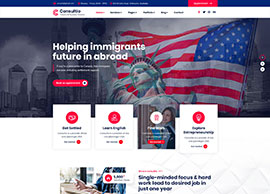
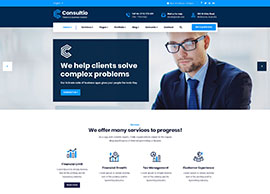
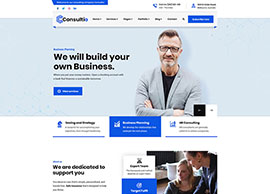
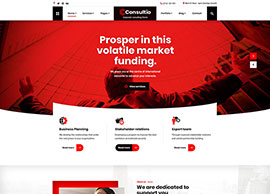
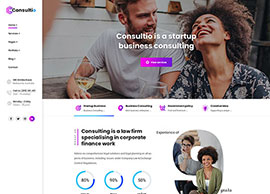
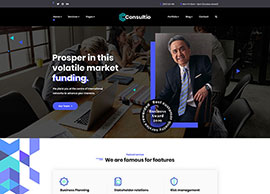
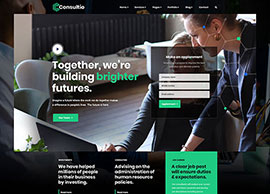
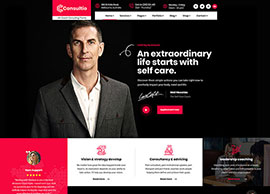
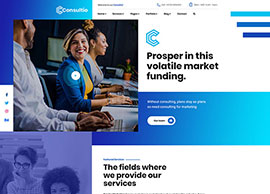
Services
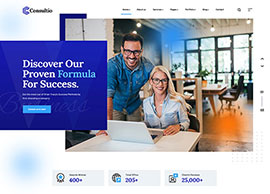
We position our clients at the forefront of their field by advancing an agenda.
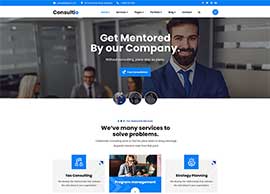
Easily apply to multiple jobs with one click! Quick Apply shows you recommended jobs based off your most recent search and allows you to apply to 25+ jobs in a matter of seconds!

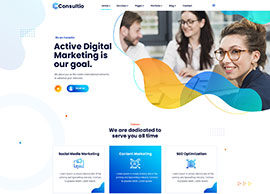
Social Media Marketing
We understand the importance of approaching each work integrally and believe in the power of simple and easy...

Content Marketing
Your logo is the very heart of your identity, let our designers deliver the perfect & dreamy design, make a...

SEO Optimization
What separates mori from all other web design agencies is ability to offer the most Friendly Experience you can...

Web Development
Increase social reach and productivity with our App Directory, a collection of famous applications like...

Media Promotion
Rounding up a bunch of specific designs & talking about the merits of each is perfect way to find common ground...

Penalty Recovery
At its core, every brand has something special to reveal something that inspires people. We are an agency...