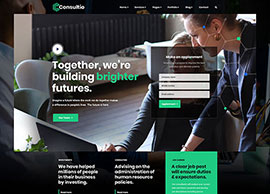
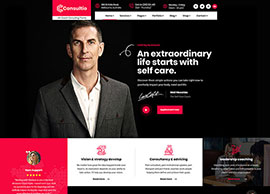
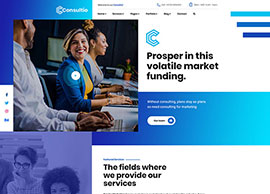
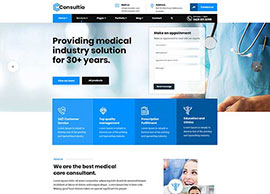
BIS Registration Consultants For Adapters
BIS Registration Consultants for Adapters – ACPL BIS Registration Consultants for Adapters – ACPL If you’re making or importing power adapters, chargers, or similar electronics, you already know the rules are strict. The Bureau of Indian Standards (BIS) says you need BIS certification before you can sell these products in…