Diagnostic Registration in India
Diagnostic Registration in India Accredited Consultants Pvt. Ltd., Simplifying Regulatory Approvals In India, diagnostic kits, reagents, and in-vitro diagnostic (IVD)…
Read MoreRegulatory updates, compliance insights, and industry analysis from the ACPL team.

Diagnostic Registration in India Accredited Consultants Pvt. Ltd., Simplifying Regulatory Approvals In India, diagnostic kits, reagents, and in-vitro diagnostic (IVD)…
Read More
ACPL Company, Your Regulatory Compliance Partner The Central Drugs Standard Control Organization (CDSCO) is India’s national regulatory authority for drugs,…
Read More
New Drug and Medical Registration in India ACPL Company, Your Trusted Regulatory Partner Bringing a new drug or medical device…
Read MoreBest Pharmacovigilance Services Provider in Noida End-to-End Drug Safety and Compliance Solutions We at ACPL provide exhaustive Pharmacovigilance (PV) services…
Read More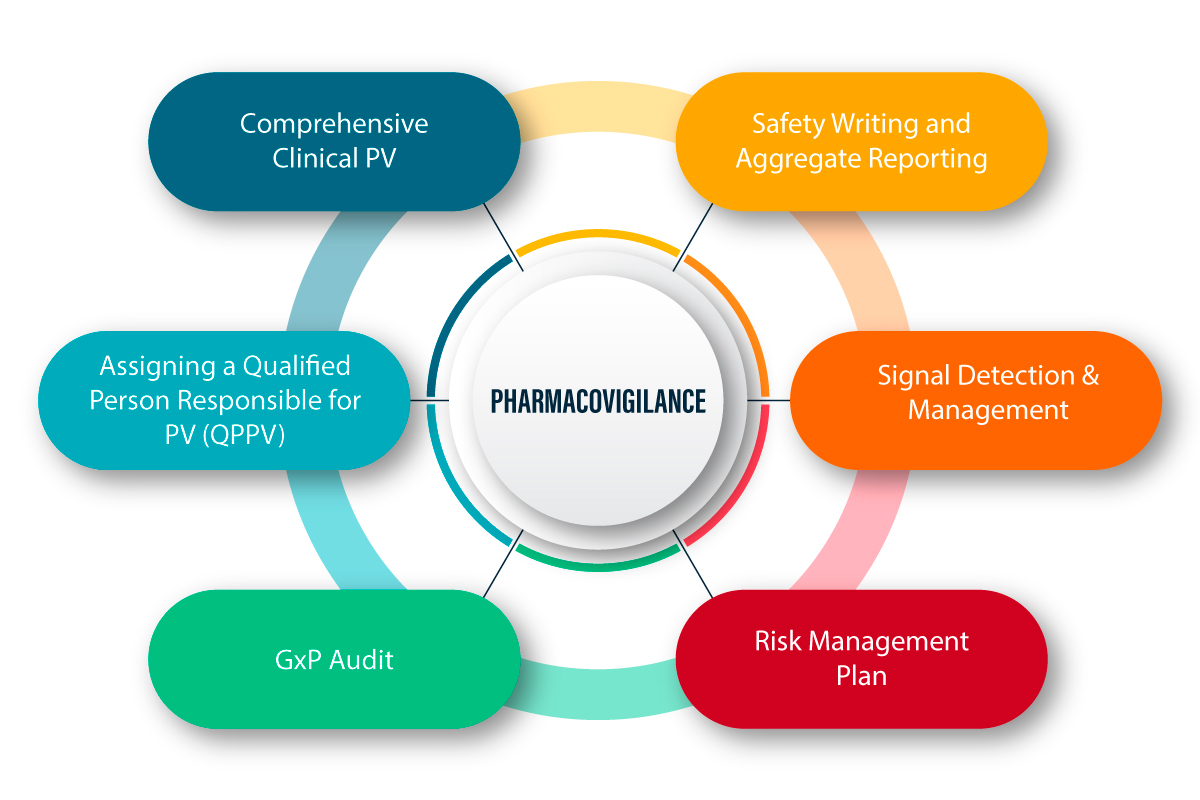
Best Pharmacovigilance Services Provider in India End-to-End Drug Safety and Compliance Solutions We at ACPL provide exhaustive Pharmacovigilance (PV) services…
Read More
What is FSSAI Food License in India ? FSSAI License India means the permission or authorization by Food Safety Standards…
Read More
FSSAI License Provider in Noida – Your One-Stop Solution for Food Business Compliance Opening or operating a food business in…
Read More
FSSAI License Provider in Noida – Your One-Stop Solution for Food Business Compliance Starting or running a food business in…
Read MoreFood License Consultants in Noida We are Food License Consultants in Noida Food Licensing & Registration System (FLRS) is an…
Read MoreExpert criminal Metrology representative in Noida – make sure full Compliance with ACPL In nowadays tremendously regulated market, compliance with…
Read More